More than 120 clinical trials underway to find Covid-19 vaccine
<p>Information and data on Covid-19 are always changing (countries affected, spread, number of people hospitalized, in critical condition and the mortality rate, etc.) and, fortunately, so is the number of scientific advances and medical solutions to tackle this new coronavirus. Let’s have a look at them.</p>

Yesterday we covered the initiatives underway in Catalonia in this regard, and in this post we’re going to look at the clinical trials that have kicked off all over the world since the beginning of the first outbreak: more than 120 in just two and a half months. The figures keep rising and China is leading the ranking, with over half of all the studies underway.
On December 31, 2019, the World Health Organization (WHO) was notified of an outbreak of “pneumonia of unknown cause” detected in the city of Wuhan, Hubei province (China), a city with 11 million inhabitants. On January 10, 2020, genomic sequencing determined that it was the novel Wuhan coronavirus, 2019-nCoV, a betacoronavirus related to MERS-CoV and SARS-CoV.
By the end of that month, more than 11,000 cases had been detected and on March 11, the WHO declared the outbreak a pandemic just days after it passed the mark of 100,000 registered cases. As of March 19, 2020, with more than 220,000 cases in 159 countries, there are 121 clinical trials registered on Clinicaltrials.gov.
By country, China has stepped up the pace and has nearly 60 trials open. They are followed by the United States, France and Hong Kong. Catalonia is on the map with the first study in Spain, a collaboration of the Catalan Ministry of Health, Catalan Institute of Health, researchers at the Fight AIDS Foundation at Hospital Germans Trias and pharmaceutical companies like Gebro and Laboratoris Rubió.
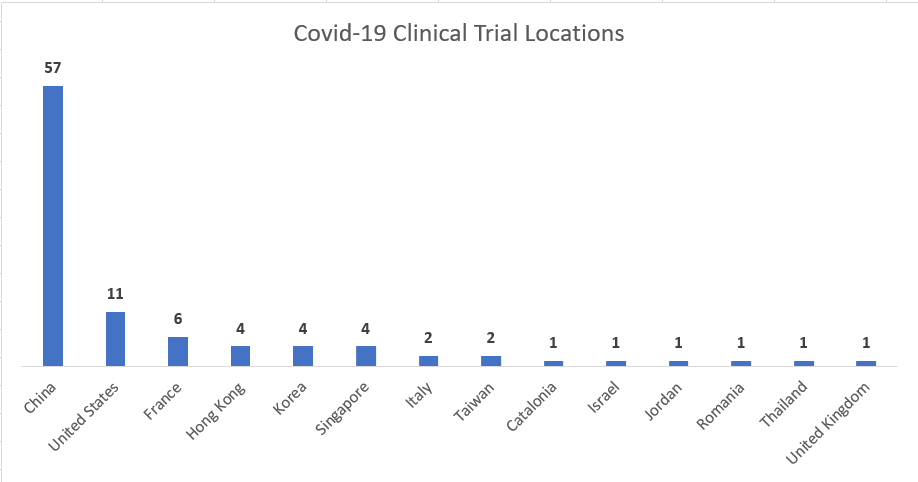
Fig. 1 Source: Clinicaltrials.gov
The Asian country has been leading the scientific battle to find a vaccine or antivirals for this novel coronavirus from the beginning. In early January, with more than 11,000 cases in its territory, it already had 10 trials underway.
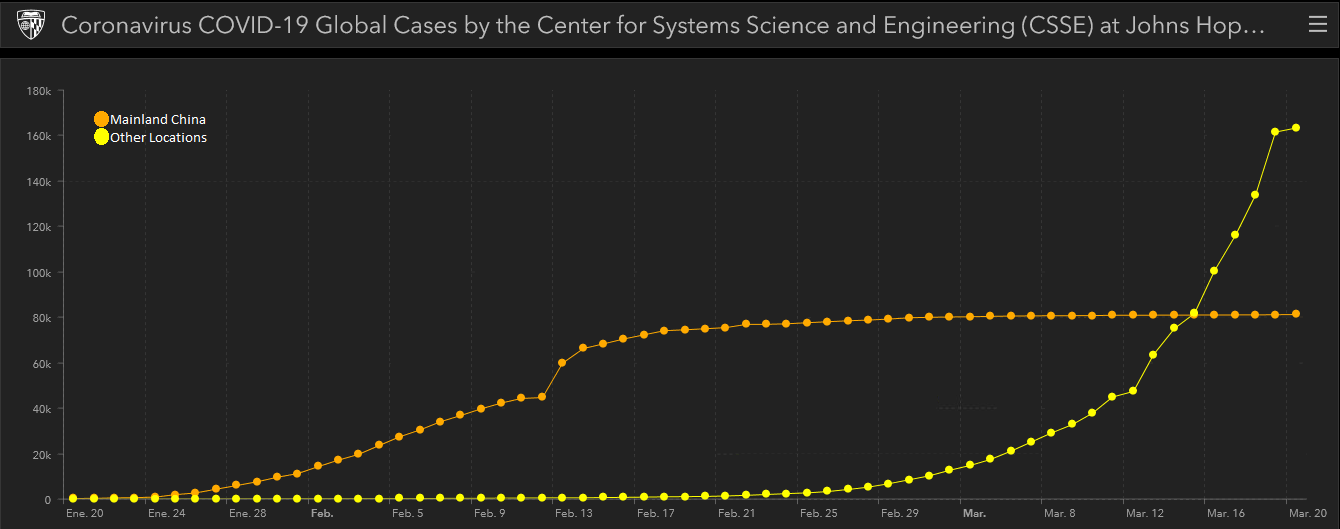
Fig. 2 Source: Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)
The graph taken from the Johns Hopkins University interactive map shows how the virus didn’t really begin to spread beyond the Asian continent until the end of February. So, as the days have passed and more countries have been affected, research groups and pharmaceutical corporations all over the world have turned their efforts towards speeding development of a new therapy.
If we look at the data on a timeline, figure 3 shows that China already had patients registered for a study in China (the first cases were in early December), and it isn’t until mid-February that other countries began to join the initiative. As the virus and the disease have spread around the world, the United States, Hong Kong, South Korea and France have joined the map.
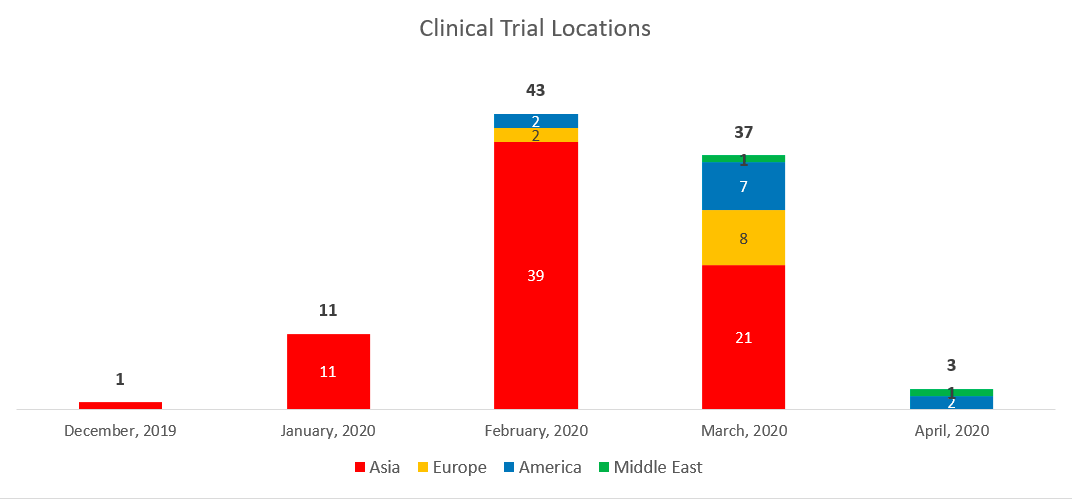
Fig. 3 Source: Clinicaltrials.gov
Although developing a solution is a long process in terms of time, human resources and production, as well as being very costly, the global spread of the problem has presented the whole world with a challenge that has all countries working in the same direction. It is undoubtedly a unique global health threat, but the number of Covid-19 clinical trials is growing every day, and this indicator makes us sure that, through science, we will win this unprecedented battle.


